Rucete ✏ Chemistry In a Nutshell
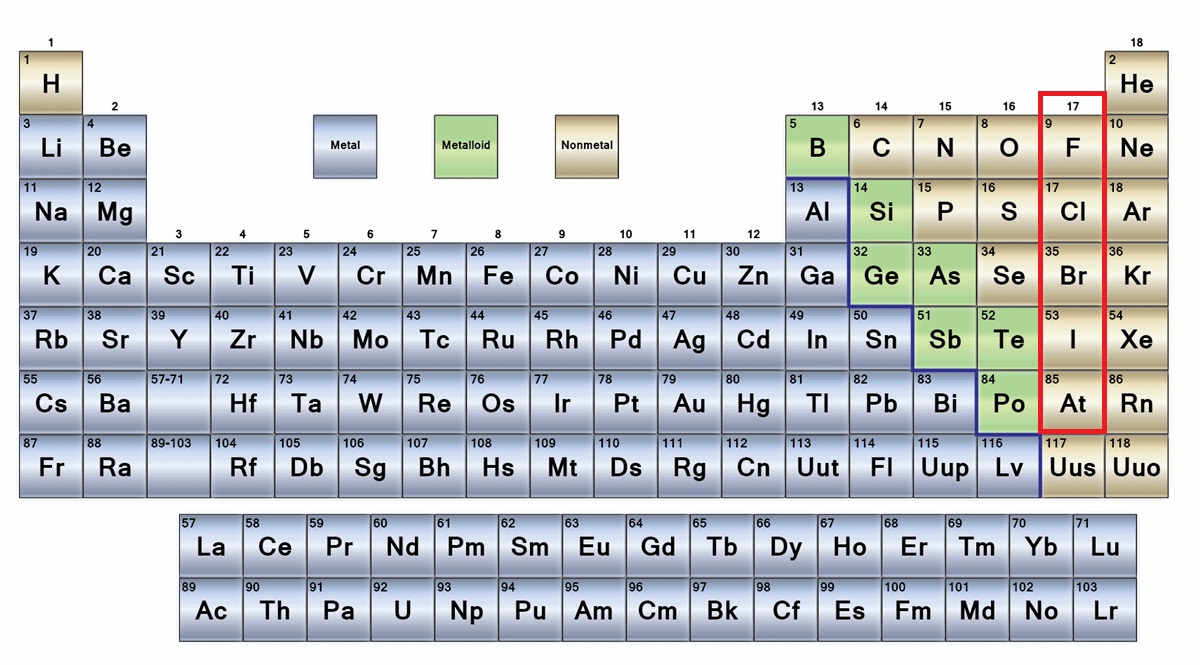
Metals vs Nonmetals
Metal
- Good conductor of heat and electricity
- Show metallic luster
- Malleable
- Ductile
- Form oxides that may react with water to give hydroxides
- From binary metal hydrides that, if soluble, are strong bases
- Have one to five electrons in outermost shell, usually not more than three.
- Readily form cations by loss of electron
- Increases metallic character form top to bottom within a group
- Decreases metallic behavior from left to right across a periodic
- Metallic behavior decreases as the positive oxidation number of the element in its compound increases
Nonmetal
- Generally poor conductor
- Generally show no metallic luster
- Usually brittle and non ductile in the solid state
- Oxidize elemental metals and often oxidize other nonmetals that have less ability to attract electrons
- Form oxides that may react with water to gives acids.
- Form acidic hydroxyl compounds (oxyacids)
- React with O2, F2, H2 and other nonmetals, giving convalent compounds
- Form binary hydrides that be acidic
- React with metals, often giving ionic
- Usually have four to eight electrons in utmost shell
- Nonmetallic feather decreases going down a group
- Increases nonmetallic character form left to right across a period
- Nonmetallic behavior increases as the positive oxidation number of the element in its compound increases
Tags:
Chemistry in a nutshell