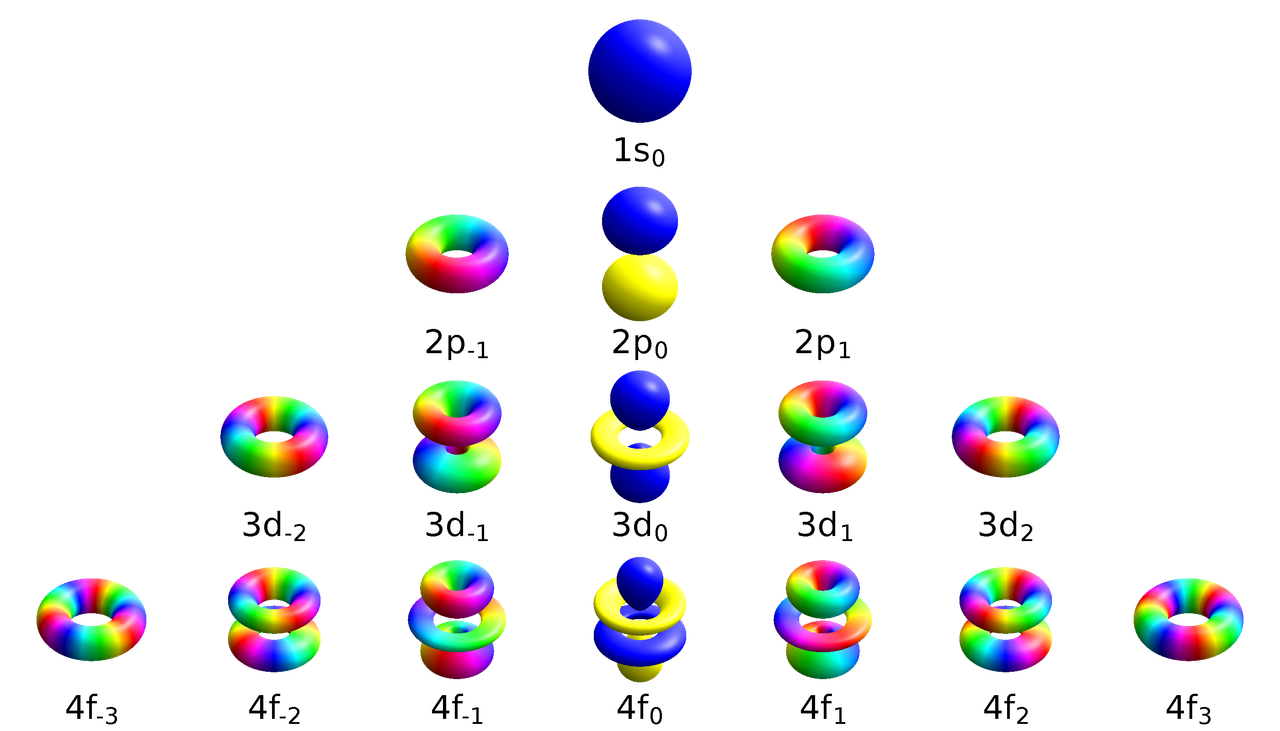Rucete ✏ Chemistry In a Nutshell
Rutherford predicted that there was a nucleus in the atom, and this model of the atom was discovered in 1911.
Atom
The etymology of atom, atom, is that it was a new word coined by adding "temnein," which means to cut into the Greek prefix "a-," which means not, as in atomos. In other words, it referred to something that was no longer cuttable. It entered ancient France as atome and eventually became an atom of English.
This concept of 'a microscopic component of the world' was not tested experimentally. It was used ideologically only in philosophy and theological reasoning, and was purely fictitious until nineteenth-century physics established the existence of atoms. Even after evidence for the existence of atoms became available, many people remained skeptical of the concept.
Currently, the atomic model uses the quantum mechanics model at the far right of the models shown in the figure above.
A brief summary of the representative atomic model is as follows.
John Dalton's atomic model
Dalton published it in 1803 as an argument for the following atomic theory.
1. All matter is composed of atoms.
2. The identical elements have the identical dimensions, masses, and properties.
3. Atoms are no longer splittable.
4. An atom cannot be converted into another atom, lost, or created.
5. The chemical reaction modifies only the method by which atoms bond to other atoms; it modifies no other atoms. As a result, the mass is retained.
The current atomic theory, on the other hand, explains why three of Dalton's five atomic theories are incorrect.
To begin, protons, neutrons, and electrons can be separated from atoms. Additionally, fission and fusion can produce as many isotopes as possible. Isotopes share similar properties but have different masses.
Due to the limitations of observation equipment, this simple model has remained firmly in place for approximately 100 years.
Thomson atomic model
Raisin pudding model. Pudding is + and raisin is -charge.
Thompson discovered the presence of electrons through cathode ray experiments, and this atomic structure was born in 1904.
He determined the mass of cathode rays and discovered that they were 1800 times lighter than hydrogen, the lightest atom, despite the fact that they resembled particles.
When electrodes are attached to both ends of a vacuum glass tube and a high voltage of thousands of volts is applied, a small amount of light is emitted from - to +, which is referred to as a cathode line. And as a result of determining whether it has a charge, it discovered that it bends toward the charge +, implying that cathode ray particles have a -charge. You now understand that this is the particle that conducts the current to the electron. Additionally, we propose a raisin pudding model to explain why electrons charged with - remain attached to atoms. Charges and -charges can be precisely offset while maintaining the stability of atoms. But soon this theory is broken by Rutherford's experiment.
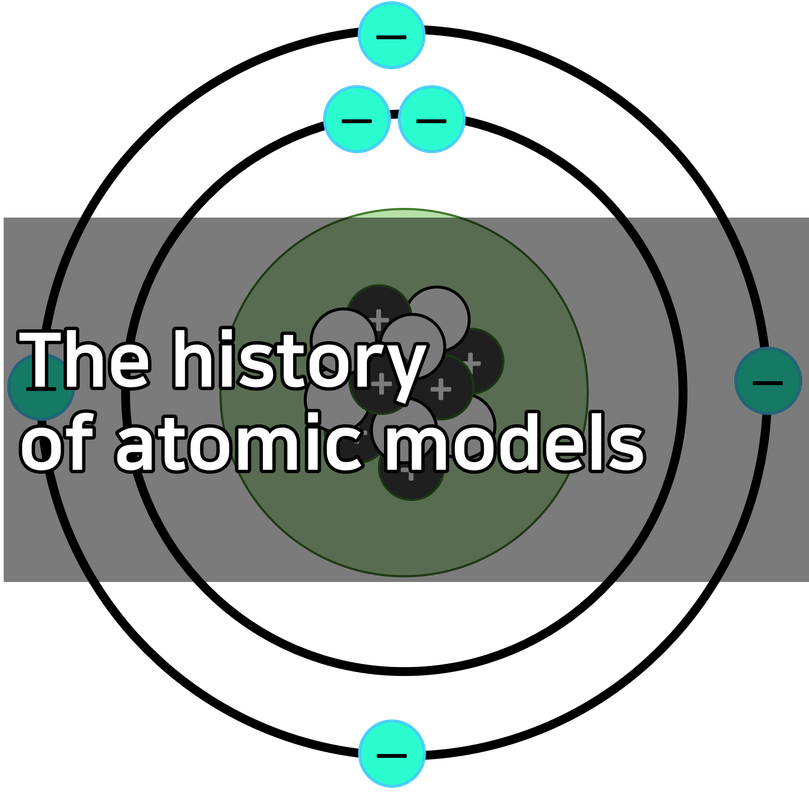
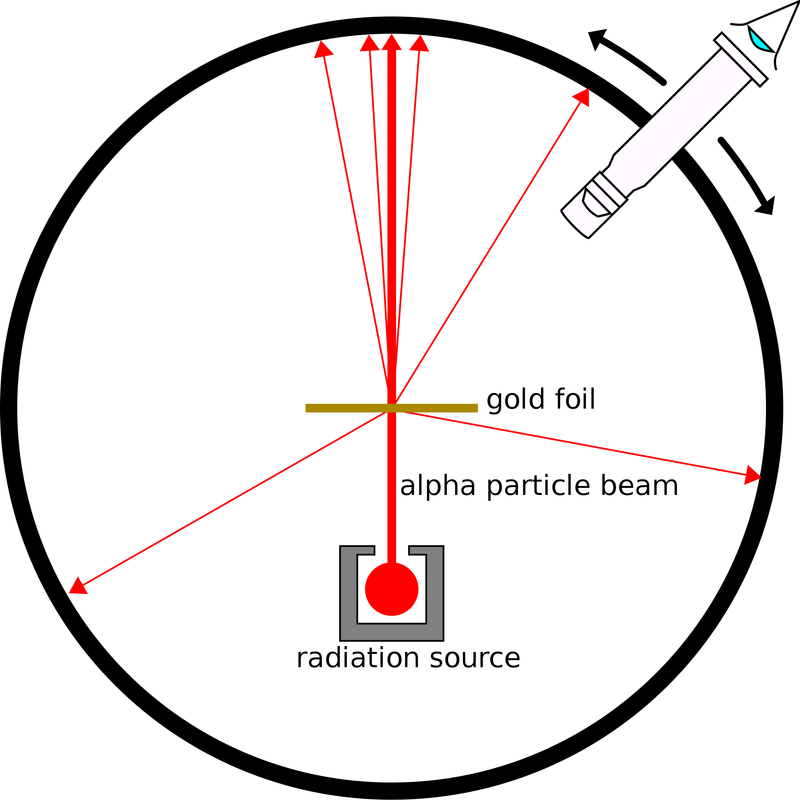
Rutherford model
Rutherford predicted that there was a nucleus in the atom, and this model of the atom was discovered in 1911.
The existence of atomic nuclei was discovered by the Geiger-Masden experiment, which is famous for Rutherford's Alpha Scattering Experiment.
Bohr atomic model
Bohr argued that his atomic model quantized the electron orbit.
In other words, the world is digital, not analog.
According to Bohr, there exists a minimum orbit in which electrons can never fall. And when it receives energy, it "momentsarily moves" into a higher orbit; when it releases energy, it teleports into a lower orbit. This is referred to as a quantum leap, and the electromagnetic waves generated at this point appear in our eyes as "light," which is the definition of "light."
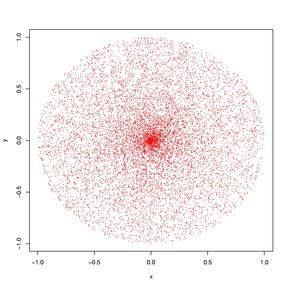
Quantum mechanical model
According to this model, the electron is now defined as the probability that it is no longer in the correct place at the correct time and is in its proper location, and this probability distribution will soon manifest as an electron cloud. Additionally, electrons are distributed along each atom's 'orbital.'
Tags:
Chemistry in a nutshell