Rucete ✏ Biology In a Nutshell
Functional Groups | In a nutshell
Many organic molecules have functional groups.
Each functional group confers on organic molecules properties such as acidity and polarity.
Hydroxyl(-OH) : polarity, hydrophilic
e.g. Ethanol, Glycerol, Sugars
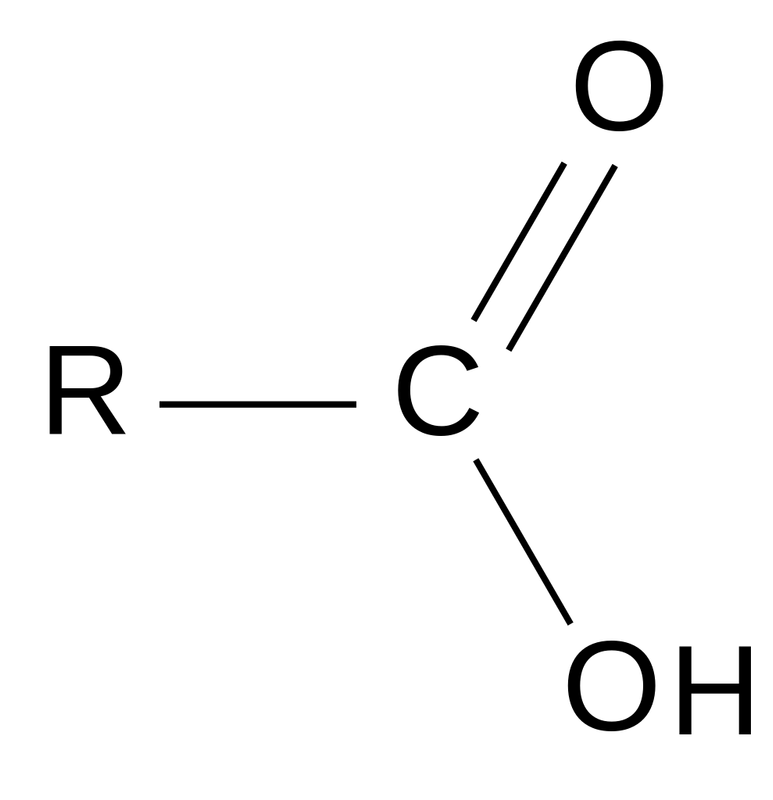
Carboxyl(-COOH) : polarity, hydrophilic, weak acid
e.g. Ethanoic acid, Amino acid, fatty acid
Amino(-NH₂) : polarity, hydrophilic, weak base
e.g. Amino acid
Phosphate(-PO₃²⁻) : polarity, hydrophilic, acid
e.g. ATP, DNA, Phospholipids
Carbonyl(-CO) : polarity, hydrophilic
e.g. Propanone, Sugars
Carbonyl(-CHO) : polarity, hydrophilic
e.g. Methanal, Sugars
Methyl(-CH₃) : non-polarity, hydrophobic
e.g. fatty acid, oil
Important functional groups
Hydrogen (-H)
Hydrogen is either polar or nonpolar, depending on the atom to which it is bonded; it is involved in condensation and hydrolysis.
Hydroxyl (-OH)
This functional group has polar properties; it is involved in condensation and hydrolysis.
Carboxyl (-COOH)
It is acidic, negatively charged when H+ dissociates, involved in peptide bonds
Amino (NH₂)
It is basic and has the potential to bond an additional H+, thereby becoming positively charged and becoming involved in peptide bonds.
Methyl (CH₃)
Phosphate (H₂SO₄)
It is acidic, and when H+ dissociates, it can produce up to two negative charges, establishes a connection between the nucleotides in nucleic acids, can act as an energy carrier group in ATP.
Tags:
Biology in a nutshell